-
Global
standardization
of medical AI -
There have been various long-term attempts to apply AI to the field of healthcare, one of the fundamental rights of humans. The exponential acceleration of data accumulation in recent years, an essential requirement for the application of AI and the related technical developments, is driving the growth of the medical AI market as well as the expansion of its utilization. Let’s examine some of the major global issues regarding the utilization of AI in healthcare and what we need to do to facilitate the medical AI industry in Korea.
Medical AI technology is currently considered an important solution to such issues as the ever increasing burden of medical expenses, medical accidents, risks in intensive care units, low levels of trust in emergency medical services, inadequate access to healthcare, and the shortage of medical professionals.
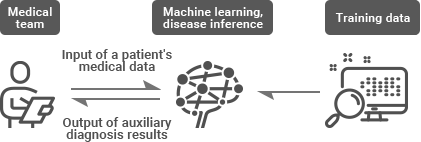
In addition, it enables timely treatment by boosting speed and accuracy in reading medical images and predicting or diagnosing disease based on historically accumulated medical data, and therefore it is expected to serve as the catalyst in reducing the time taken to develop new drugs, resolving tough healthcare issues, reducing medical expenses, and improving the overall quality of healthcare services.
So how is the standardization of AI progressing in both Korea and the wider world? JTC 1, the joint technical committee established by the ISO (International Organization for Standardization) and the IEC (International Electrotechnical Commission), has been working on the standardization of basic IT for about twenty years. The ISO/IEC JTC 1 has contributed significant efforts to the development of standards in fields related with AI, such as AI-based language, machine-human interaction, biometrics, computer-based image processing, cloud computing, big data, and sensor networks.
Furthermore, the WHO (World Health Organization) and the ITU (International Telecommunication Union) have been promoting discussions on methods and procedures related with such issues as resolving the difficulty of interpretability and explainability, guaranteeing trustworthiness and robustness, and the need to protect the privacy of medical data required for training and performance evaluation, all of which have been raised in the process of applying AI to healthcare.
At the ITU-T SG16 meeting held in Ljubljana, Slovenia in July 2018 with the aim of identifying issues related with standardization, the participants reached an agreement on the need to establish the FG-AI4H (Focus Group on AI for Health), which would remain active for two years.
-

FG-AI4H(Focus Group on AI for Health)A group focusing on the development of a
standardized method of evaluating AI-based
healthcare solutions and algorithms.

-
01
A future healthcare technology analyzing
a patient’s condition via a hologram.
-
Medical 3D printing
and its standardization
in Korea -
The WG12 formed in October 2017 under JTC1 with the remit of establishing standards for 3D printing and scanning is also working on AI standards. It aims to devise the fundamental standards for 3D printing and scanning by combining different ICTs, as well as applied standards for 3D printing and scanning in such fields as healthcare, defense and DIY.
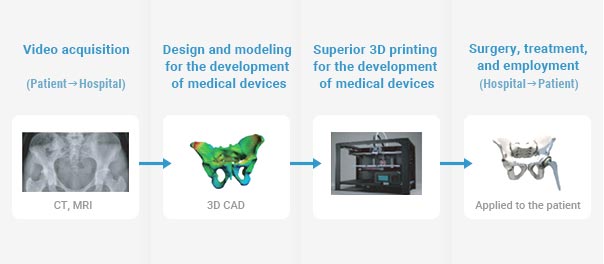
Medical 3D printing requires the building of a 3D model based on medical images and video clips of patients obtained through 2D/3D conversion. This field requires the strictest possible standards based on deep learning, as each hospital uses different 3D printing software as well as different technologies for splitting and optimizing video clips.
In particular, medical 3D printing for modeling and producing devices and fixtures for dental clinics and transplanting, surgical guides and tailored braces, etc. requires processing specifically designed for medical purposes. As such, diverse standards will be developed with deep learning technology.
It is natural to expect that other deep learning-based technological standards aimed at optimizing a variety of 3D models will be developed, such as structural mechanics-based analysis, a point cloud-based 3D scanning format, and 3D mesh optimization.
-
Meanwhile, no standards have been developed or announced specifically for medical AI, which means that they will have to be developed along with methods of screening and authorizing new ones. Also, there is an urgent need to respond to international standards, which have been quickly emerging around the world, at the local level. The current lack of a definitive standard or specifications for AI-based medical devices has forced the KFDA to establish and operate its own, including screening methods.
As an ever increasing number of AI-based medical devices flood the market, there will be a much greater need to harmonize the standards and specifications of different countries at the international level, as well as to develop an internationally accepted standard.
-
Guidelines for screening and approval of
medical devices equipped with big data
and AI technologies

-
Innovating and
distributing
Korean AI technologies -
AI technologies have undergone surprisingly dramatic developments. Each national government is providing all-out support and multinational conglomerates are making omni-directional investments. Just as Masayoshi Son, the chairperson of Softbank, emphasized the importance of AI by saying “The first (focus) is AI, the second is also AI, and the third is also AI,” so the strategic investment in AI technologies is becoming more important and significant than ever before. In particular, we need to pay close attention to whatever China does in this respect.
In July 2017, the Premier of the State Council of China announced his country's “Next-generation AI Development Plan”, which sets out a comprehensive roadmap for the country to develop into a global AI powerhouse by 2030. Also, the “Three-year Action Plan to Promote the Next-generation AI Industry”, announced in December 2017, provides detailed implementation strategies for all-round investments across China in a unified manner.
The “Regulations for the Development of Next-generation AI” mentions the need to establish standards for AI technologies as an essential element in the propagation of the AI industry. The country announced that it will set up AI-related technical standards that cover such matters as fundamental commonality, mutual connection, industrial application, online security, and privacy protection.
The “Three-year Action Plan” also announced its core mission as facilitating the development of AI-related products, along with the goals of developing and commercializing diagnosis technologies for diseases of the brain, lungs, eyes, bones, cardiovascular system, and breasts, as well as developing an auxiliary diagnosis system for AI-based medical imaging with a false-negative rate of less than 1% and a false-positive rate of less than 5% by 2020.
In order to ensure the quality of these products and technologies, China plans to set up an AI industry support system by 2020 as one of its action goals, along with establishing the high-quality database resources of a certain scale, developing and opening a standard measurement database, and developing an AI standard system, an analysis and evaluation system, and a safety guarantee system as a default.
-

JTC 1
joint technical committee 1
WG12
Working Group 12 under JTC1
-
02
National strategies are urgently needed,
along with organized policy support
and cooperation, to invigorate the related
industries and to promote innovation
and the proliferation of medical AI
technologies. 
-
Thanks to these comprehensive strategic investment efforts, China is witnessing the explosive growth of diverse medical AI technologies and related services. For example, the country is operating "One-minute Clinics” where patients can measure their blood pressure and temperature in a 3㎡ “unmanned examination room” (which costs as little as KRW 5 million) and inform the AI doctor of their symptoms via the video display, while a human doctor in a remote location may ask some additional questions based on this information and recommend medications accordingly.
Numerous applications and services based on medical AI will emerge as the U.S. FDA has approved more than forty medical AI products and services, while its Korean counterpart has authorized about ten products from Vuno, Lunit an JLK.
Furthermore, as the FDA is pondering how to set up a system for approving medical AI products in a more accelerated manner in light of the strong market demand, Korea has to adjust its current standards and approval system to conform with international harmonization and regulations in order to facilitate the domestic medical AI industry. As we can see from the AI development case of China, standardization is the core foundation needed for sustainable growth as well as the most essential and supportive element.
In order to enable the innovation and proliferation of medical AI technologies in Korea, it will be necessary to implement national strategies derived from a comprehensive and mid-long standards system in tandem with organized policy support, invigoration of the related industries, and cooperation.

Author · Senior Researcher Jong-hong Jeon / Chief Gang-chan Lee, Intelligence and Information Industry Standard Research Lab
This article has been taken from Issue 5, Volume 34 of Electronic Communication Trend Analysis published by ETRI.